- 0shares
- Facebook0
- Twitter0
- Pinterest0
- LinkedIn0

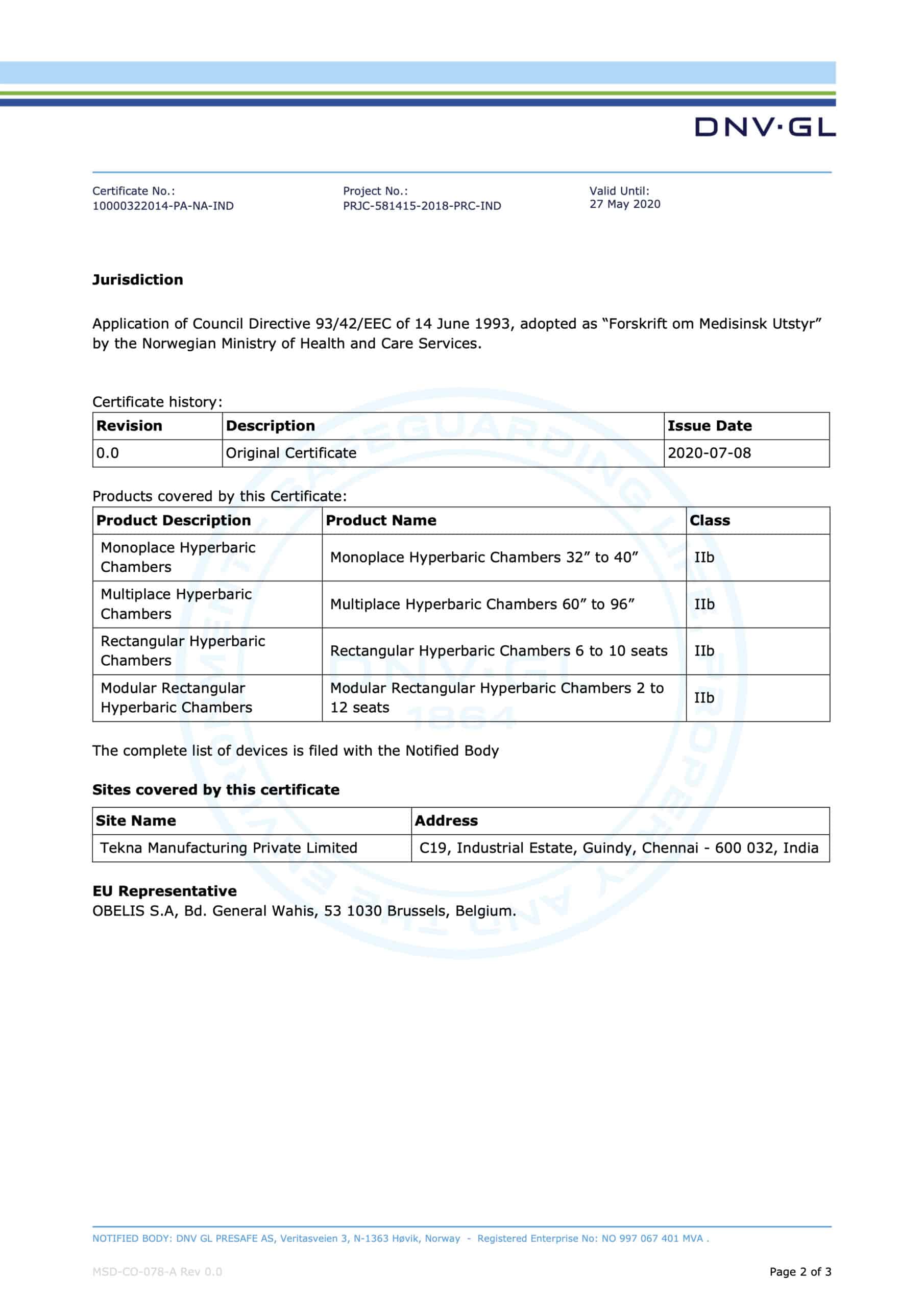

EC CERTIFICATE
Full Quality Assurance System
Certificate No.: Project No.: Valid Until:
10000322014-PA-NA-IND PRJC-581415-2018-PRC-IND
This is to certify that the quality system of:
27 May 2024
Tekna Manufacturing Private Limited
C19, Industrial Estate, Guindy, Chennai – 600 032, India.
For design, production and final product inspection/testing of:
HYPERBARIC CHAMBERS
Has been assessed with respect to:
THE CONFORMITY ASSESSMENT PROCEDURE DESCRIBED IN ANNEX II EXCLUDING SECTION 4 OF COUNCIL DIRECTIVE 93/42/EEC ON MEDICAL DEVICES, AS AMENDED
and found to comply.
Further details of the product(s) and conditions for certification are given overleaf.
Place and date:
Høvik, 08 July 2020
For:
DNV GL PRESAFE AS Notified Body No.: 2460
______________________________________________________________________________
Mariann Jeremiassen
The certificate is digitally verified by blockchain technology. For more info, see www.dnvgl.com/assurance/certificates-in-the- blockchain.html
PROD 021
Notice: The Certificate is subject to terms and conditions as set out in the Certification Agreement. Failure to comply may render this Certificate invalid.
NOTIFIED BODY: DNV GL PRESAFE AS, Veritasveien 3, N-1363 Høvik, Norway – Registered Enterprise No: NO 997 067 401 MVA .
MSD-CO-078-A Rev 0.0 Page 1 of 3
Certificate No.: Project No.: Valid Until:
10000322014-PA-NA-IND PRJC-581415-2018-PRC-IND
Jurisdiction
27 May 2020
Application of Council Directive 93/42/EEC of 14 June 1993, adopted as “Forskrift om Medisinsk Utstyr” by the Norwegian Ministry of Health and Care Services.
Certificate history:
Revision Description
0.0 Original Certificate Products covered by this Certificate:
Issue Date
2020-07-08
Class
IIb
Product Description
Monoplace Hyperbaric Chambers
Multiplace Hyperbaric Chambers
Rectangular Hyperbaric Chambers
Modular Rectangular Hyperbaric Chambers
Product Name
Monoplace Hyperbaric Chambers 32” to 40” Multiplace Hyperbaric Chambers 60” to 96”
IIb Rectangular Hyperbaric Chambers 6 to 10 seats IIb
Modular Rectangular Hyperbaric Chambers 2 to 12 seats
IIb
Tekna Manufacturing Private Limited C19, Industrial Estate, Guindy, Chennai – 600 032, India
EU Representative
OBELIS S.A, Bd. General Wahis, 53 1030 Brussels, Belgium.
The complete list of devices is filed with the Notified Body
Sites covered by this certificate
Site Name Address
NOTIFIED BODY: DNV GL PRESAFE AS, Veritasveien 3, N-1363 Høvik, Norway – Registered Enterprise No: NO 997 067 401 MVA .
MSD-CO-078-A Rev 0.0 Page 2 of 3
Certificate No.: Project No.: Valid Until:
10000322014-PA-NA-IND PRJC-581415-2018-PRC-IND
Terms and conditions
27 May 2020
The certificate is subject to the following terms and conditions:
-
Any producer (see 2001/95/EC for a precise definition) is liable for damage caused by a defect in his
product(s), in accordance with directive 85/374/EEC, as amended, concerning liability of defective
products.
-
The certificate is only valid for the products and/or manufacturing premises listed above.
-
The Manufacturer shall fulfil the obligations arising out of the quality system as approved and uphold
it so that it remains adequate and efficient.
-
The Manufacturer shall inform Presafe of any intended updating of the quality system and Presafe will
assess the changes and decide if the certificate remains valid.
-
Periodical audits will be held, in order to verify that the Manufacturer maintains and applies the
quality system. Presafe reserves the right, on a spot basis or based on suspicion, to pay unannounced visits.
The following may render this Certificate invalid: Changes in the quality system affecting production.
Periodical audits not held within the allowed time window.Conformity declaration and marking of product
When meeting with the terms and conditions above, the producer may draw up an EC declaration of conformity and legally affix the CE mark followed by the Notified Body identification number of Presafe.
End of Certificate
NOTIFIED BODY: DNV GL PRESAFE AS, Veritasveien 3, N-1363 Høvik, Norway – Registered Enterprise No: NO 997 067 401 MVA .
MSD-CO-078-A Rev 0.0 Page 3 of 3
- 0shares
- Facebook0
- Twitter0
- Pinterest0
- LinkedIn0
